T. +44 (0) 1283 224337 E. info@surepharm.com
Analysis & Development


ANALYSIS >
PRODUCT DEVELOPMENT >
" Surepharm extends beyond the traditional roles of QA and QC, providing analytical support services and stability studies "
- Product Analysis: our GMP-accredited analytical laboratory, as a matter of course, will provide full chemical and physical testing, including release testing, utilising a wide array of modern instrumentational and classical analytical techniques. This is all performed in-house by our experienced team
- Bespoke Analytical Support: our laboratory can also provide a wide range of support services, including custom test method development and validation
- Stability Studies: an essential part of bringing new products to market. Surepharm has a wealth of experience in diligently managing this process for our partners. This includes ICH compliant studies, study design, sample preparation / packaging and formulation stability advice, to support products intended for a global market
- GMP-accredited analytical laboratory providing full chemical and physical testing, performed in-house by our experienced team
- We employ both modern, instrumentational and classical analysis methodologies
- Bespoke analytical support, including custom test method development and validation
- ICH-compliant stability studies
- Specialist in solid dose analysis
" Drawing upon years of experience, we can provide customers with tailormade product development services "
- Formulation Development: a standard service for customers in partnership with Surepharm, including a closely-managed process of New Product Introduction. Together with years of solid dose and technology transfer experience, this provides peace-of-mind and an efficient route to market for brand new formulations, or in the optimisation of existing products. We have dedicated facilities and a team in our Pilot Plant with the expertise to bring your concept to life
- Pilot Batches: our Pilot Plant is home to a dedicated team of development technicians and is equipped at a small scale to match the facilities available in full scale manufacture. Our experienced staff will manage the process from small scale (to address regulatory needs) right through to the supervision of the first commercial validation batches, with a continuity of care and attention to detail our partners have come to expect and enjoy
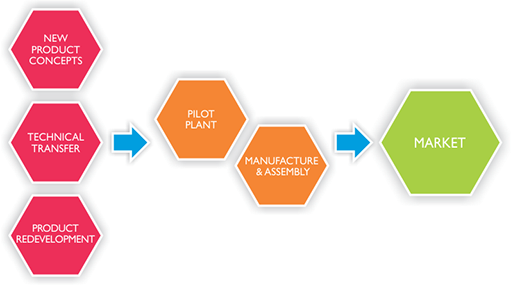
In the unceasing pursuit of higher standards, the Technical Department at Surepharm extends beyond the traditional roles of QA and QC, providing analytical support services, formulation development facilities, management of pilot batches and stability studies.
"Surepharm offers a complete contract manufacturing service from formulation development to distribution with the experience and flexibility to meet your needs"
OUR COMMITMENT
At Surepharm we endeavour to exceed the expectations of our customers, utilising our wide experience to attain the highest levels of product quality. Our goal remains to be the preferred specialist solid dose manufacturer, within the pharmaceutical sector.
If you have any questions about our products or services, please do not hesitate to contact us.
CONTACT DETAILS
Surepharm Services Ltd.
Bretby Business Park
Ashby Road, Bretby
Burton on Trent, Staffordshire
DE15 0YZ. United Kingdom
Telephone Support +44 (0) 1283 224337
© Surepharm Services Ltd.
